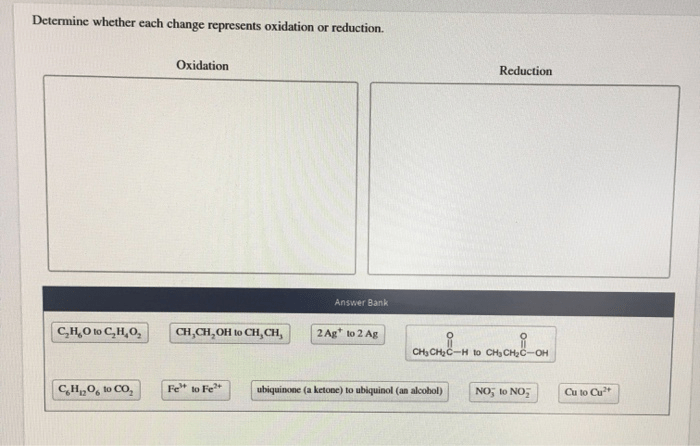
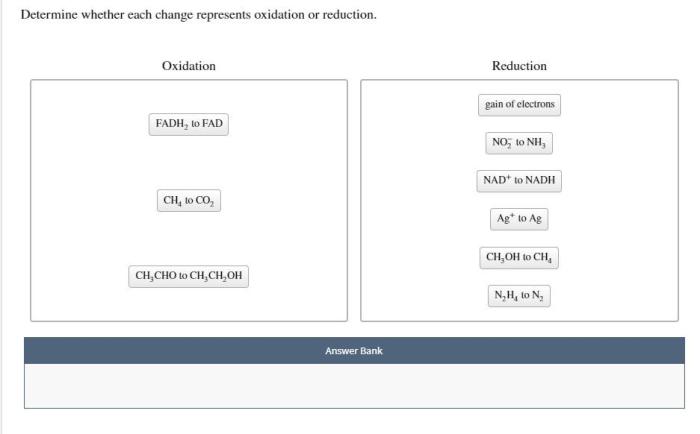
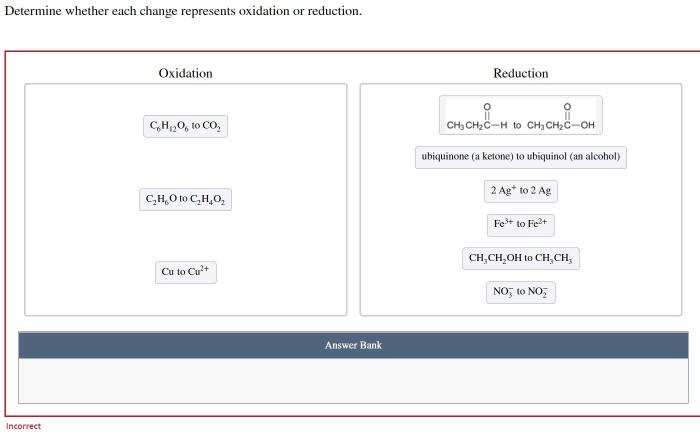
Determine whether each change represents oxidation or reduction. – In the realm of chemistry, oxidation and reduction reactions play a pivotal role. Understanding the ability to determine whether each change represents oxidation or reduction is crucial for comprehending the intricate dance of electrons in chemical transformations. This comprehensive guide delves into the concepts, criteria, and methods involved in identifying oxidation and reduction, providing a foundation for exploring their significance in diverse scientific fields.
The concept of oxidation and reduction, often abbreviated as redox reactions, involves the transfer of electrons between atoms or ions. Oxidation refers to the loss of electrons, while reduction signifies the gain of electrons. Recognizing these changes is essential for unraveling the mechanisms and predicting the outcomes of chemical reactions.
Understanding Oxidation and Reduction
Oxidation and reduction are fundamental chemical processes involving the transfer of electrons between atoms or ions. Oxidation refers to the loss of electrons, while reduction refers to the gain of electrons.
Examples of oxidation include the rusting of iron and the burning of fuels. In the rusting process, iron atoms lose electrons to oxygen, forming iron oxide. In combustion, carbon atoms in the fuel lose electrons to oxygen, forming carbon dioxide.
Identifying Oxidation and Reduction in Changes
To determine whether a change represents oxidation or reduction, the oxidation number of each element involved must be considered. Oxidation number is a measure of the electron charge an atom possesses or appears to possess in a compound.
An increase in oxidation number indicates oxidation, while a decrease indicates reduction. Oxidation numbers can be assigned using a set of rules, and they are essential for understanding the chemical changes that occur in redox reactions.
Methods for Determining Oxidation and Reduction, Determine whether each change represents oxidation or reduction.
- Half-Reaction Method:This method involves separating the overall reaction into two half-reactions, one for oxidation and one for reduction. The species that is oxidized increases in oxidation number, while the species that is reduced decreases in oxidation number.
- Redox Tables:Redox tables provide a list of common oxidation-reduction couples and their corresponding standard reduction potentials. By comparing the standard reduction potentials of the reactants and products, it is possible to determine which species is oxidized and which is reduced.
Table of Oxidation and Reduction Reactions: Determine Whether Each Change Represents Oxidation Or Reduction.
| Reactants | Products | Oxidation/Reduction |
|---|---|---|
| Fe + O2 | Fe2O3 | Fe: Oxidation, O: Reduction |
| CH4 + 2O2 | CO2 + 2H2O | C: Oxidation, O: Reduction |
| 2Cu2+ + 4I– | 2CuI + I2 | Cu: Reduction, I: Oxidation |
Applications of Identifying Oxidation and Reduction
Identifying oxidation and reduction is crucial in various fields:
- Chemistry:It helps in understanding chemical reactions, predicting product formation, and balancing redox equations.
- Biology:It plays a vital role in cellular respiration, photosynthesis, and other metabolic processes.
- Environmental Science:It aids in understanding environmental processes such as corrosion, air pollution, and water treatment.
Essential Questionnaire
What is the fundamental difference between oxidation and reduction?
Oxidation involves the loss of electrons, whereas reduction entails the gain of electrons.
How can I determine the oxidation number of an element?
The oxidation number represents the hypothetical charge an atom would have if all bonds to other atoms were ionic. It can be determined using specific rules and guidelines.
What is the significance of redox reactions in biological systems?
Redox reactions are crucial in biological processes, such as cellular respiration and photosynthesis, where electron transfer drives energy production and metabolic pathways.