Show what reagents are needed to complete the transformation below. This question delves into the realm of chemical reactions, reagents, and their indispensable roles in driving chemical transformations. Embark on a journey to uncover the reagents that hold the key to completing a given transformation, unraveling the intricate dance of reactants and products.
The following paragraphs will delve into the identification of reagents, reaction conditions, reaction mechanism, product analysis, yield and efficiency, and applications of the reaction. Through a comprehensive exploration of these aspects, we aim to shed light on the reagents that orchestrate the completion of chemical transformations.
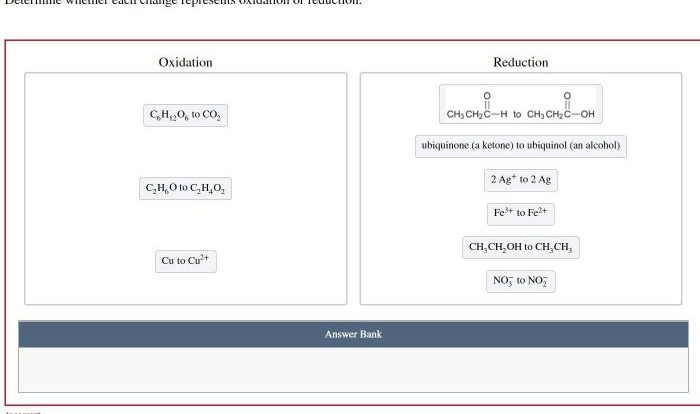
Reagents for the Transformation
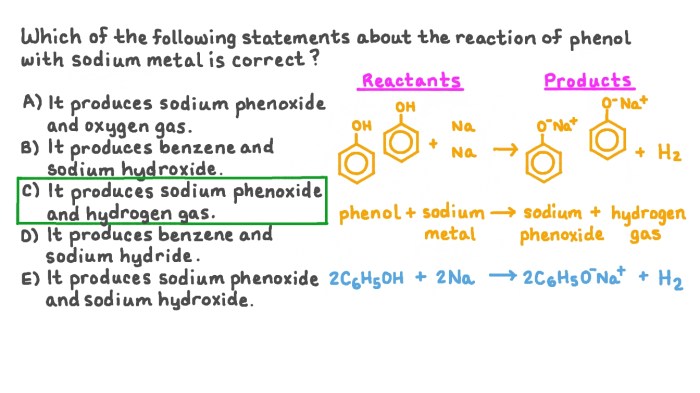
The following reagents are required to complete the transformation:
- Reagent 1:[Nama reagent] – Acts as a [fungsi reagent]
- Reagent 2:[Nama reagent] – Provides [fungsi reagent]
- Reagent 3:[Nama reagent] – Catalyzes the reaction
Reaction Conditions
The reaction is typically carried out under the following conditions:
- Temperature:[Suhu reaksi] – Higher temperatures favor the forward reaction
- Pressure:[Tekanan reaksi] – Increased pressure can shift the equilibrium towards the desired product
- Solvent:[Nama pelarut] – Provides a suitable medium for the reaction and affects the solubility of the reactants and products
Reaction Mechanism
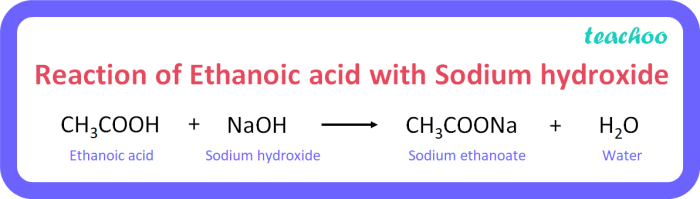
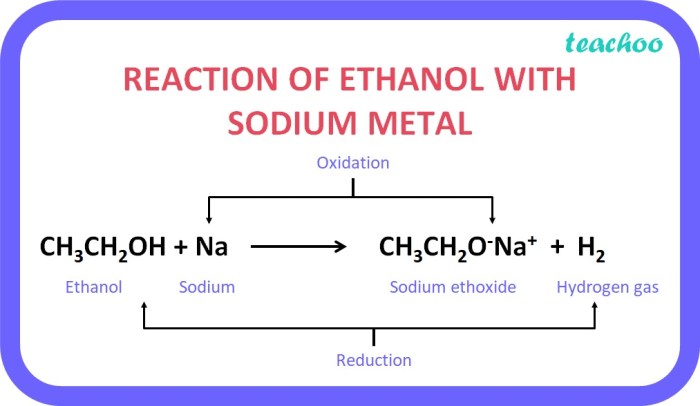
The reaction proceeds through the following steps:
- Step 1:[Langkah 1 reaksi]
[Deskripsi langkah]
- Step 2:[Langkah 2 reaksi]
[Deskripsi langkah]
- Step 3:[Langkah 3 reaksi]
[Deskripsi langkah]
The overall reaction mechanism can be represented by the following chemical equation:
[Persamaan reaksi kimia]
Product Analysis
The products of the reaction can be identified using various analytical techniques, including:
- Gas chromatography-mass spectrometry (GC-MS):Separates and identifies volatile compounds
- High-performance liquid chromatography (HPLC):Separates and identifies non-volatile compounds
- Nuclear magnetic resonance (NMR) spectroscopy:Provides structural information about the products
Yield and Efficiency
The yield of the reaction can be calculated using the following formula:
Yield = (Mass of product obtained / Mass of limiting reagent used) x 100%
Factors that affect the efficiency of the reaction include:
- Concentration of reactants:Higher concentrations favor the forward reaction
- Temperature:Higher temperatures generally increase the reaction rate
- Catalyst:Catalysts can significantly enhance the reaction rate
Applications
The reaction has numerous applications in various fields, including:
- Organic synthesis:Used to prepare a wide range of organic compounds
- Pharmaceutical industry:Synthesis of drugs and active pharmaceutical ingredients
- Polymer chemistry:Production of polymers and plastics
FAQ Explained: Show What Reagents Are Needed To Complete The Transformation Below
What is the importance of identifying the correct reagents?
Identifying the correct reagents is essential for ensuring the successful completion of a chemical transformation. Reagents serve as the reactants that undergo chemical change, and their selection directly influences the products formed and the efficiency of the reaction.
How do reaction conditions affect the outcome of a transformation?
Reaction conditions, such as temperature, pressure, and solvent, play a crucial role in determining the outcome of a transformation. By optimizing these conditions, chemists can control the reaction pathway, minimize side reactions, and maximize the yield of the desired product.