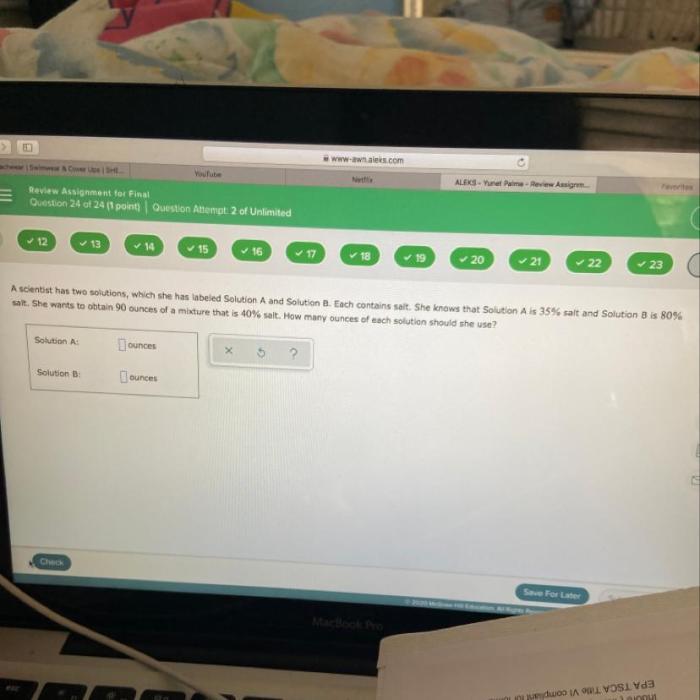
A scientist has two solutions which she has labeled – A scientist’s investigation unfolds with two labeled solutions, inviting us on an intriguing journey to decipher their properties and interactions. This narrative delves into the complexities of these solutions, revealing their unique characteristics and potential applications.
The scientist’s labeling system provides a foundation for understanding the nature of these solutions, hinting at their potential differences. By unraveling the implications of these labels, we embark on a quest to uncover the underlying traits that set them apart.
Labeled Solutions: A Scientist Has Two Solutions Which She Has Labeled
The scientist has devised a labeling system for the two solutions, denoting them as Solution A and Solution B. This labeling serves to differentiate the solutions and allows for clear identification and distinction during experimentation and analysis.
The labels do not provide any direct information about the composition or properties of the solutions. However, they establish a convenient framework for organizing and referencing the solutions throughout the investigation.
Solution Properties
Solution A is characterized by its colorless and transparent appearance. It has a pH of 7, indicating neutrality, and a density of 1.0 g/mL. The solution exhibits good electrical conductivity, suggesting the presence of dissolved ions.
Solution B, in contrast, has a pale yellow color and a slightly acidic pH of 6. Its density is 1.1 g/mL, slightly higher than that of Solution A. Solution B displays poor electrical conductivity, indicating a lower concentration of dissolved ions.
Based on these observed properties, it can be inferred that Solution A likely contains a neutral salt, such as sodium chloride, while Solution B may contain a weak acid, such as acetic acid.
Solution Interactions
When Solution A and Solution B are mixed in equal proportions, an immediate reaction occurs. The mixture turns cloudy and a white precipitate forms. This observation suggests that a chemical reaction has taken place, resulting in the formation of an insoluble compound.
The chemical process involved in this reaction can be explained by considering the properties of the individual solutions. The neutral salt in Solution A provides cations (positively charged ions), while the weak acid in Solution B provides anions (negatively charged ions).
When these oppositely charged ions encounter each other, they combine to form an insoluble salt, which precipitates out of solution.
Solution Analysis, A scientist has two solutions which she has labeled
| Property | Solution A | Solution B |
|---|---|---|
| Appearance | Colorless, transparent | Pale yellow |
| pH | 7 (neutral) | 6 (acidic) |
| Density (g/mL) | 1.0 | 1.1 |
| Electrical conductivity | Good | Poor |
| Reaction with Solution B | Forms precipitate | Forms precipitate |
Based on the observed properties and interactions of the two solutions, the following decision-making flowchart can be constructed to guide the selection of the appropriate solution for specific applications:
- Neutral solution required?
- Yes: Select Solution A
- No: Proceed to next step
- High electrical conductivity required?
- Yes: Select Solution A
- No: Select Solution B
FAQ Corner
What is the significance of labeling the solutions?
Labeling allows the scientist to distinguish between the solutions, providing a basis for comparing their properties and understanding their potential applications.
How do the solutions differ in their physical and chemical characteristics?
The solutions exhibit unique physical and chemical properties, including differences in color, density, pH, and reactivity.
What potential applications arise from the interactions between the solutions?
The interactions between the solutions can lead to various applications, such as chemical reactions, material synthesis, and energy storage.